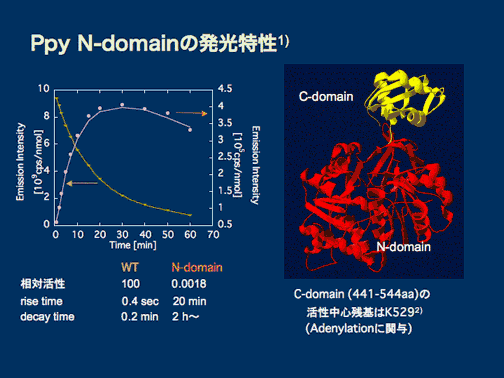
We found that although weak (and slow), the N-terminal domain alone has its luminescence activity using the two substrates, and also found that we can specifically and sensitively detect the reaction intermediate LH2-AMP in the presence of excess luciferin and ATP by using this domain based on the different kinetics. This enabled us to measure the LH2-AMP concentration during the reactions of various mutants, which is expected to lead to the elucidation of the not fully understood reaction mechanism. Also expected is the development of protein-protein interaction detection method based on the mutant enzymes (FlimPIA).
References
1) T. Zako et.al. (2003) Biochim. Biophys. Acta 1649, 183-189.
2) K. Ayabe et.al. (2005) FEBS Lett.. 579, 4389-4394.
3) Y. Ohmuro-Matsuyama, et al. (2013) Anal. Chem. 85, 7935-7940.[DOI][Abstract]
4) Press release by JST (2013)
5) Y. Ohmuro-Matsuyama, et al. (2014) Anal. Chem. 86, 2013-2018. [DOI][Abstract]